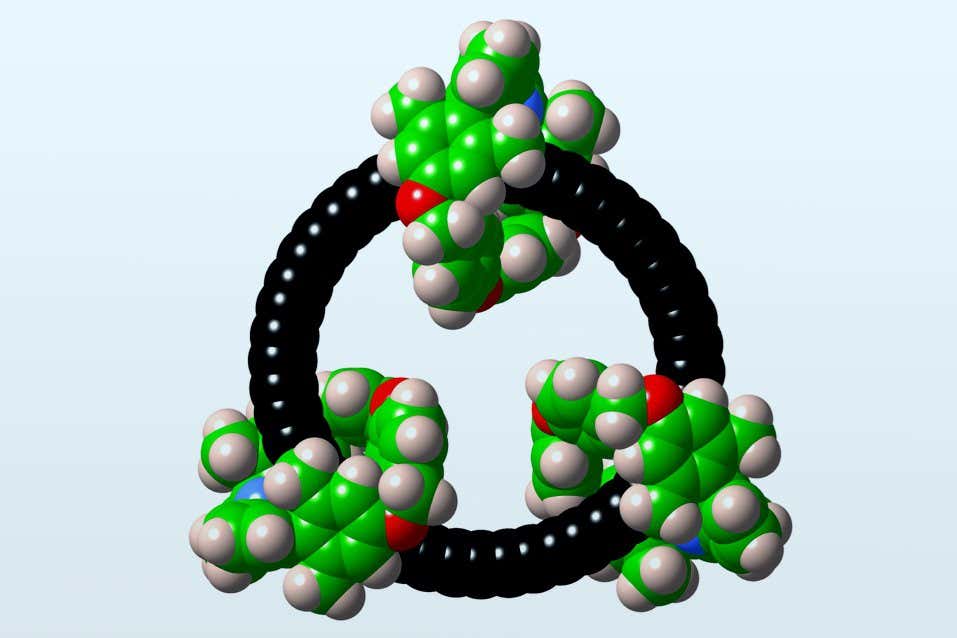
Researchers stabilised a ring-shaped carbon molecule by adding “bumpers” to protect its atoms
Harry Anderson
A new type of all-carbon molecule has been studied under normal room-temperature conditions. This marks only the second time this has ever been done, after spherical buckyballs were synthesised 35 years ago. The breakthrough could lead to extremely efficient materials for new electronic and quantum technologies.
Cyclic carbons, molecules made up of a ring of carbon atoms, could display bizarre chemical behaviour or conduct electricity in unusual ways – much like their all-carbon molecular cousins, buckyballs and nanotubes. But these rings are so delicate they usually fall apart, or in some cases even explode, before researchers have a chance to study them.
“Cyclic carbons are intriguing molecules, and we’ve been trying to make them for a long time,” says Harry Anderson at the University of Oxford. Doing so has traditionally required extremely harsh conditions in order to keep the molecules around long enough to be studied. But Anderson and his colleagues found a way to stabilise cyclic carbons at room temperature.
The technique involves modifying a cyclic carbon. The researchers demonstrated this on a never-before-studied molecule: a ring of 48 carbon atoms, called cyclo[48]carbon, or C48. Anderson and his colleagues added “bumpers” to the C48, threading it through three smaller rings, to protect the 48 atoms from colliding with each other – or with other molecules.
“There’s no unnecessary decoration,” says Max von Delius at the University of Ulm in Germany. “There’s an absolute beauty in the simplicity.”
The new structure, called cyclo[48]carbon [4]catenane, remained stable enough to study for about two days, enabling researchers to examine cyclo[48]carbon in detail for the first time. Intriguingly, the molecule’s 48 carbons acted like they were arranged in an infinite chain, a structure theoretically capable of transferring electric charge from one atom to the next indefinitely.
This possible electricity-conducting potential hints cyclic carbons could be used in a range of next-generation technologies, including transistors, solar cells, semiconductors and quantum devices. However, further research is needed to confirm this.
The new technique for stabilising cyclic carbons may also inspire other researchers to study their own exotic carbon molecules. “I think maybe there will be a race now,” says von Delius. “Think of this long ring as a stepping stone to making the infinite chain.”
A chain of single carbon molecules, von Delius explains, would make an even better conductor than a ring like C48. “This will be truly, truly amazing – and truly the next step,” he says.
Topics:
Source link : https://www.newscientist.com/article/2492719-scientists-created-a-new-carbon-molecule-for-the-second-time-ever/?utm_campaign=RSS%7CNSNS&utm_source=NSNS&utm_medium=RSS&utm_content=home
Author :
Publish date : 2025-08-14 19:00:00
Copyright for syndicated content belongs to the linked Source.