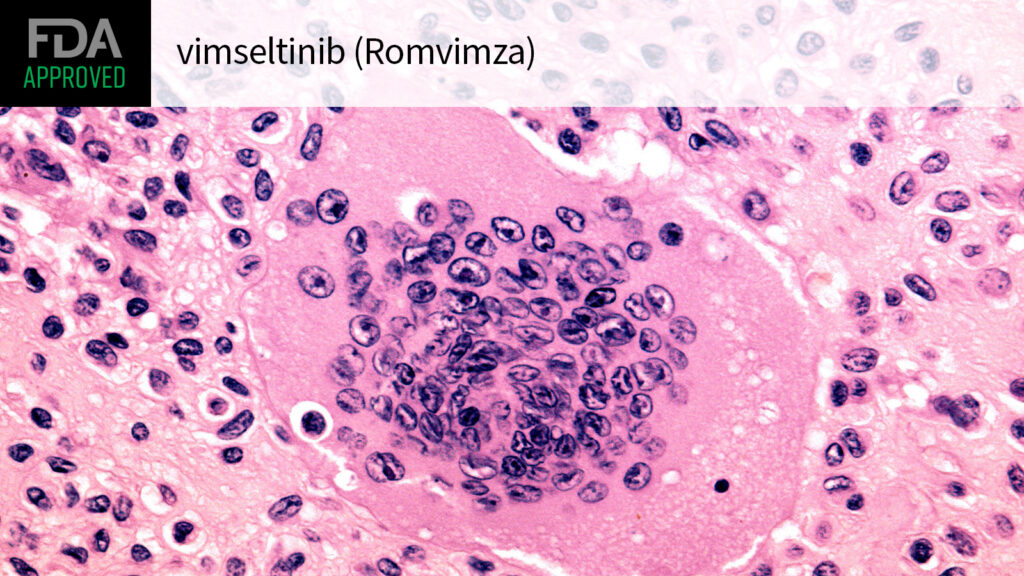
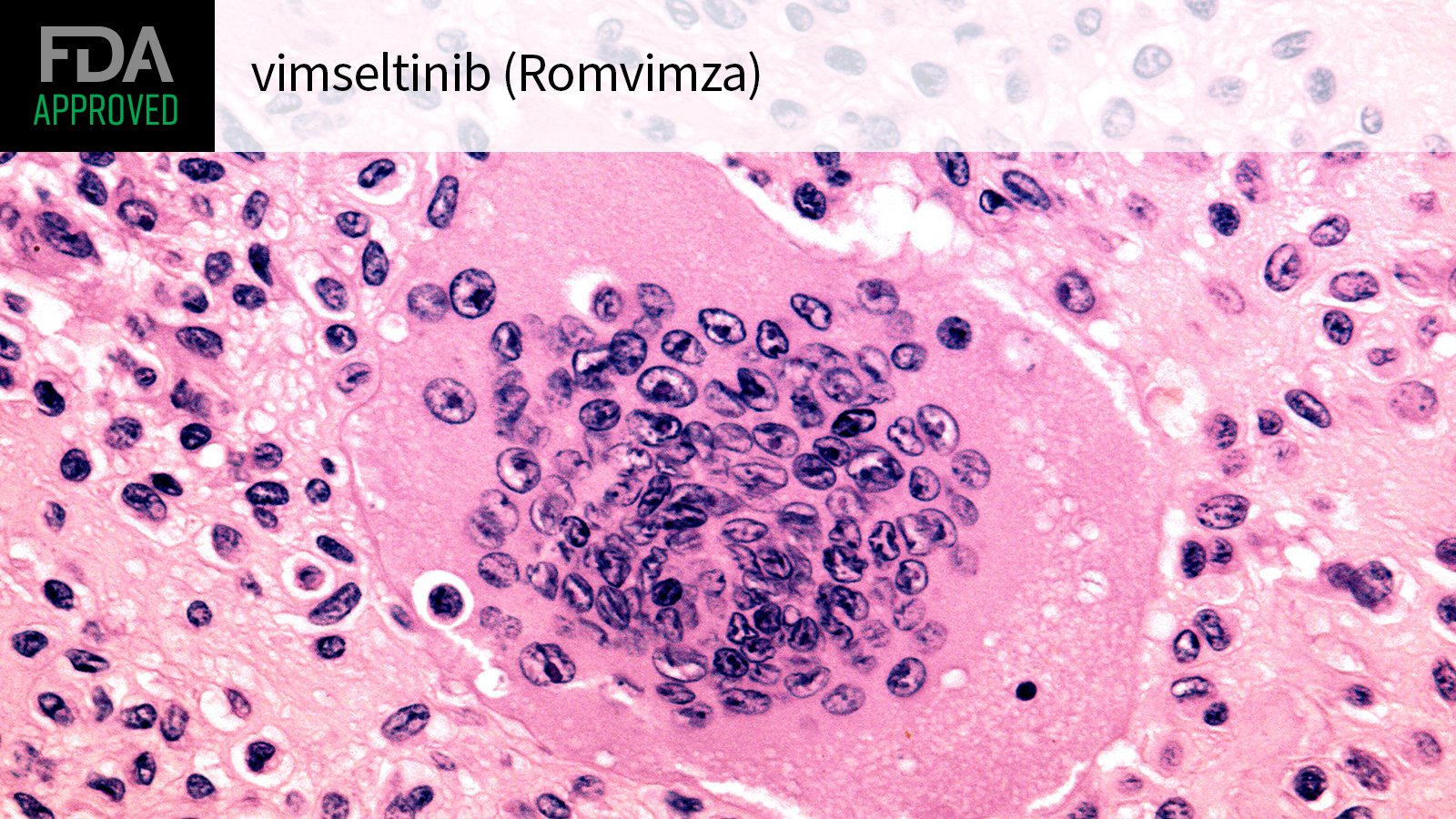
 (MedPage Today) — The FDA approved the small-molecule inhibitor vimseltinib (Romvimza) for adults with symptomatic tenosynovial giant cell tumor (TGCT), a rare, non-malignant, but debilitating, condition arising in the synovium, the agency announced…
(MedPage Today) — The FDA approved the small-molecule inhibitor vimseltinib (Romvimza) for adults with symptomatic tenosynovial giant cell tumor (TGCT), a rare, non-malignant, but debilitating, condition arising in the synovium, the agency announced…
Source link : https://www.medpagetoday.com/hematologyoncology/othercancers/114242
Author :
Publish date : 2025-02-14 22:06:43
Copyright for syndicated content belongs to the linked
Source.