TOPLINE:
Pilot study finds a non-significant 8 mmol/mol reduction in A1c following the use of intermittent scanning continuous glucose monitoring (isCGM) among adults with type 2 diabetes (T2D) on non-insulin treatment. Use of isCGM was associated with a significant 18% improvement in time in range (TIR).
METHODOLOGY:
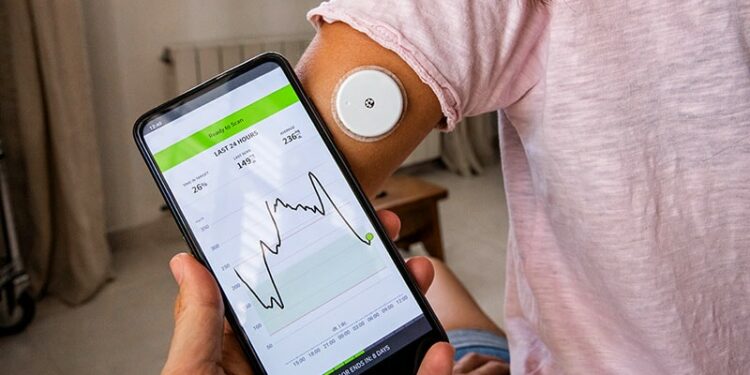
- A total of 40 adults with T2D were randomly assigned (n = 20 each) to either FreeStyle Libre 2 (Libre 2), an isCGM system, or a “blinded” CGM, the FreeStyle Libre Pro iQ (Libre Pro), for 12 weeks.
- Diabetes distress was assessed using the two-item diabetes distress scale.
TAKEAWAY:
- Overall, 53% (n = 10/19) of participants in the intervention group achieved the primary outcome, a reduction in A1c of ≥ 5.5 mmol/mol vs 35% (n = 7/20) in the control group (P = .34).
- The average TIR rose from 30% at baseline to 50% at follow-up in the isCGM group, a significant 18% increase (P = .028), compared with virtually no change in the blinded CGM group (37%-39%).
- Other CGM metrics for hypoglycaemia, hyperglycaemia, and glucose variability were comparable between study groups.
- No participants experienced an episode of hypoglycaemia according to CGM data, with zero time spent urea.
- The prevalence of high diabetes distress scale decreased from 50% at baseline to 25% at 12 weeks in the intervention groups, whereas increasing from 45% to 55% in the control groups, a non-significant difference between groups (P = .72).
- There were no serious adverse events in either group.
IN PRACTICE:
“For adults living with T2DM who are not on insulin, [the National Institute for Health and Care Excellence] does not recommend routine monitoring of blood glucose unless they are on medications that cause hypoglycaemia or have a previous history of hypoglycaemia episodes. However, with the advent of [CGM], many individuals with T2D have started monitoring their glucose levels using these devices,” the authors wrote.
“Improvement in TIR suggests isCGM could potentially have long-term benefits in this population including delaying insulin initiation. Future studies should follow participants for at least 6 months and incorporate food diaries and measurement of activity levels to further characterise the mechanism through which isCGM improves glycaemic outcomes,” they added.
SOURCE:
Conducted by Emmanuel Ssemmondo, MRCP (UK), of Allam Diabetes Centre, University of Hull, and Hull University Teaching Hospitals NHS Trust, Kingston-upon-Hull, UK, and colleagues. The study was published online on December 11, 2024, in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The 12-week trial duration may have limited the ability to observe a significant change in A1c. A small sample size.
DISCLOSURES:
The study was funded by an Association of British Diabetologists Fellowship grant. The authors have no further disclosures.
Source link : https://www.medscape.com/s/viewarticle/sensor-technology-aids-those-non-insulin-treated-t2d-2024a1000o02?src=rss
Author :
Publish date : 2024-12-20 14:00:00
Copyright for syndicated content belongs to the linked Source.