TOPLINE:
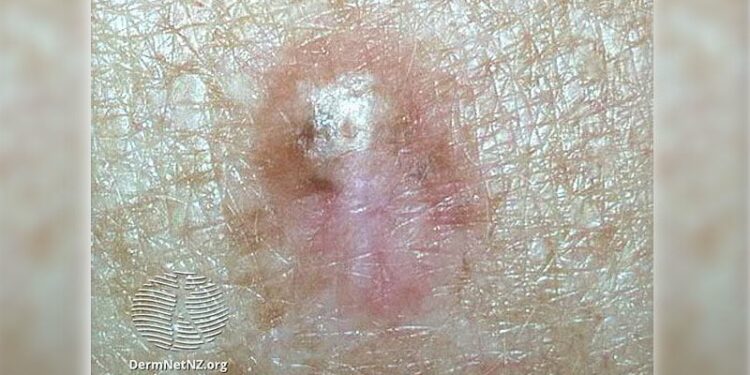
Survivors of blood or marrow transplant (BMT) face a significant risk of developing cutaneous malignant neoplasms, including basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma, with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed-up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47) compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, they noted, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, UV-light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post-BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/blood-or-marrow-transplant-recipients-face-high-risk-skin-2024a1000okj?src=rss
Author :
Publish date : 2024-12-19 12:59:38
Copyright for syndicated content belongs to the linked Source.