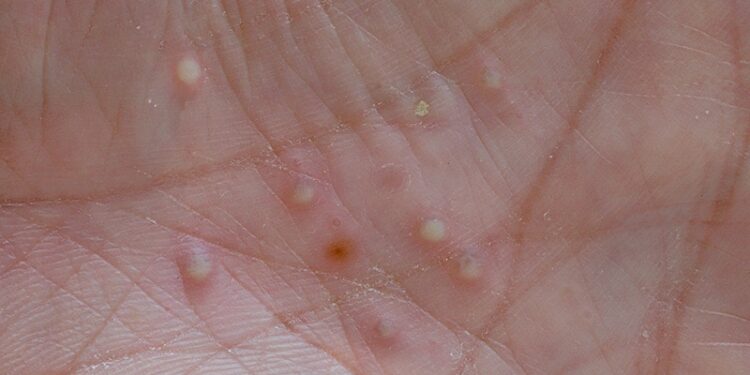
In a secondary analysis of phase 2b spesolimab data in generalized pustular psoriasis (GPP), nearly two-thirds of patients who received 300 mg monthly doses maintained clear or nearly clear skin through 48 weeks’ follow-up, and nearly one quarter of treated patients maintained minimal GPP impact on quality of life (QOL). Nevertheless, investigators said, the presence of moderate QOL impairment despite clear or nearly clear skin at baseline highlighted the chronic nature of GPP and patients’ need for effective long-term treatment. Similar observations emerged from a separate study that characterized GPP flares and treatment patterns.
Skin Symptoms and QOL
In the post hoc analysis of Effisayil 2 data, investigators led by Kenneth B. Gordon, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, found that overall, 20.0% of patients who received monthly subcutaneous spesolimab 300 mg (after a 600 mg subcutaneous loading dose) had sustained improvement in both GPP Physician Global Assessment (GPPGA) and Dermatology Life Quality Index (DLQI) scores through week 48 compared with 3.2% of the placebo cohort.
Regarding skin manifestations, 63.3% of patients treated with spesolimab experienced sustained improvement, which investigators defined as having a GPPGA score of 0/1 at all Effisayil 2 study visits through week 48 without other investigator-prescribed medications. The corresponding figure among patients on placebo was 29.0%. The study recently appeared in the Journal of the American Academy of Dermatology (JAAD).
As for QOL, 24.1% of spesolimab-treated patients maintained DLQI scores of 0/1 through week 48 vs 3.2% for placebo. Patients reported at baseline that GPP’s biggest impact on QOL stemmed from itch, soreness, and pain; embarrassment; and the disease’s effects on social activities and clothing choices.
Although patients in Effisayil 2 had GPPGA 0/1 at baseline, Gordon and colleagues noted that the mean baseline DLQI scores of 11.1 and 7.2 in the 300 mg monthly and placebo groups, respectively, indicated that despite patients’ having clear or nearly clear skin, moderate QOL burden persisted.
Spesolimab, a monoclonal antibody that blocks interleukin (IL)-36 signaling known to be involved in GPP, was first approved by the US Food and Drug Administration in 2022 for treating flares in adults with GPP. Approval was expanded in 2024 for the treatment of GPP in adults and in pediatric patients aged ≥ 12 years who weigh ≥ 40 kg.
Real-World Data
In a separate study also recently published in JAAD, investigators found that among 638 eligible patients with GPP from the OMNY Health real-world data platform, 63% experienced at least one GPP flare between January 2017 and January 2023. The mean annualized flare rate was 0.91 per patient per year, with a mean inter-flare interval of 5.9 months.
Patients who experienced flares were more likely to be women, younger than 65 years, non-White, Hispanic, or Latino, and had moderate or severe GPP and a history of infectious or parasitic disease. Treatment strategies were diverse, nonstandardized, and off-label, with patients frequently switching or discontinuing biologics and/or nonsteroidal systemic treatments.
“GPP patients continue to experience frequent flares with traditional off-label therapies in the real-world setting,” wrote authors led by Jamie W. Rhoads, MD, MS, of the University of Utah School of Medicine, Salt Lake City. Among patients who experienced multiple flares, they added, 66% had a second flare within 6 months of their first.
Together, said James G. Krueger, MD, PhD, the D. Martin Carter professor in clinical investigation and co-director of the Center for Clinical and Translational Science at The Rockefeller University, New York City, the two papers examine how patients view their health status between flares and what the frequency of reflaring might be. He was not involved with the studies but provided comments in an interview with Medscape Medical News.
Fear of Flaring
Patients worry about GPP flares, said Krueger, because such flares constitute a medical emergency with potentially severe, life-threatening symptoms that could require hospitalization for weeks while receiving traditional immunosuppressants. And GPP is so rare that a practicing dermatologist may see only a few cases in their career.
“Given that almost no center takes in this type of patient,” he added, “there’s a lack of knowledge about the natural course of GPP and how best to manage patients with this disease.”

Before spesolimab’s approval for GPP, he said, broadly acting immunosuppressants targeted GPP indirectly. The fact that doctors could quell flares using such medications did not mean that patients’ inflammation had resolved, Krueger added. “It means their inflammatory state may have been suppressed to the point that they didn’t need hospitalization. But many of these patients report significant symptoms or quality-of-life issues between flares.”
Mechanistically, Krueger said, only around one quarter of patients with GPP possess a genetic mutation in a control protein called the IL-36 receptor antagonist, which normally would prevent the overblown IL-36 response that characterizes GPP. Nevertheless, he said, the mutation spotlights the IL-36 cytokine family as the most important, consistent aberration in causing GPP.
“This is one of the instances where a rare disease has a known genetic association, and that genetic problem can be fixed to a large extent by a therapeutic antibody. This antibody essentially replaces the function of the missing protein by binding to a receptor for IL-36 in a way that prevents it from being activated.”
Accordingly, said Krueger, spesolimab approximates a molecular replacement therapy for GPP. “And I believe that’s why spesolimab works remarkably fast.”
Ultimately, Krueger said, both articles mainly address medical dermatologists at referral centers who treat patients with more complicated GPP flares. “The general practitioner should be aware that there are care pathways for acute and chronic GPP that may keep people under enough control that they can avoid recurrent emergency visits to either a dermatologist or an emergency room.”
The new information about the likelihood that a patient will have lifelong disease “helps with getting into the mindset that you’re going to need to manage most of these patients over a long time period, not episodically,” he added.
The spesolimab study was supported by Boehringer Ingelheim, maker of spesolimab; authors reported financial relationships with many companies that manufacture psoriasis drugs, including AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, and others. The second study was also supported by Boehringer Ingelheim. Rhoads had received consulting fees from Boehringer Ingelheim, Eli Lilly, and Genentech. Another author of the second study is an employee of OMNY Health, a contractor to Boehringer Ingelheim for the real-world study, and had received consulting fees from Boehringer Ingelheim, Eli Lilly, and Genentech. Two authors are Boehringer Ingelheim employees, and another author had received research grants and is a consultant to and had received honoraria from Boehringer Ingelheim. Krueger has been a consultant and lecturer for Boehringer Ingelheim and has analyzed biopsy samples from Effisayil 1 and 2 for future publications, but he is not an Effisayil author.
John Jesitus is a Denver-based medical writer and editor.
Source link : https://www.medscape.com/viewarticle/spesolimab-provides-sustained-improvement-gpp-symptoms-and-2025a1000e5e?src=rss
Author :
Publish date : 2025-05-29 12:00:00
Copyright for syndicated content belongs to the linked Source.