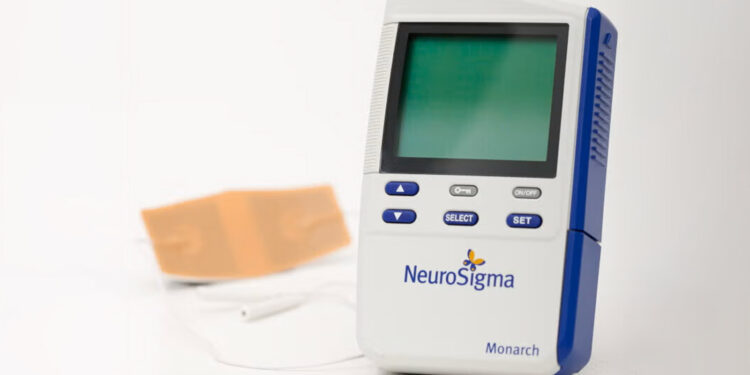
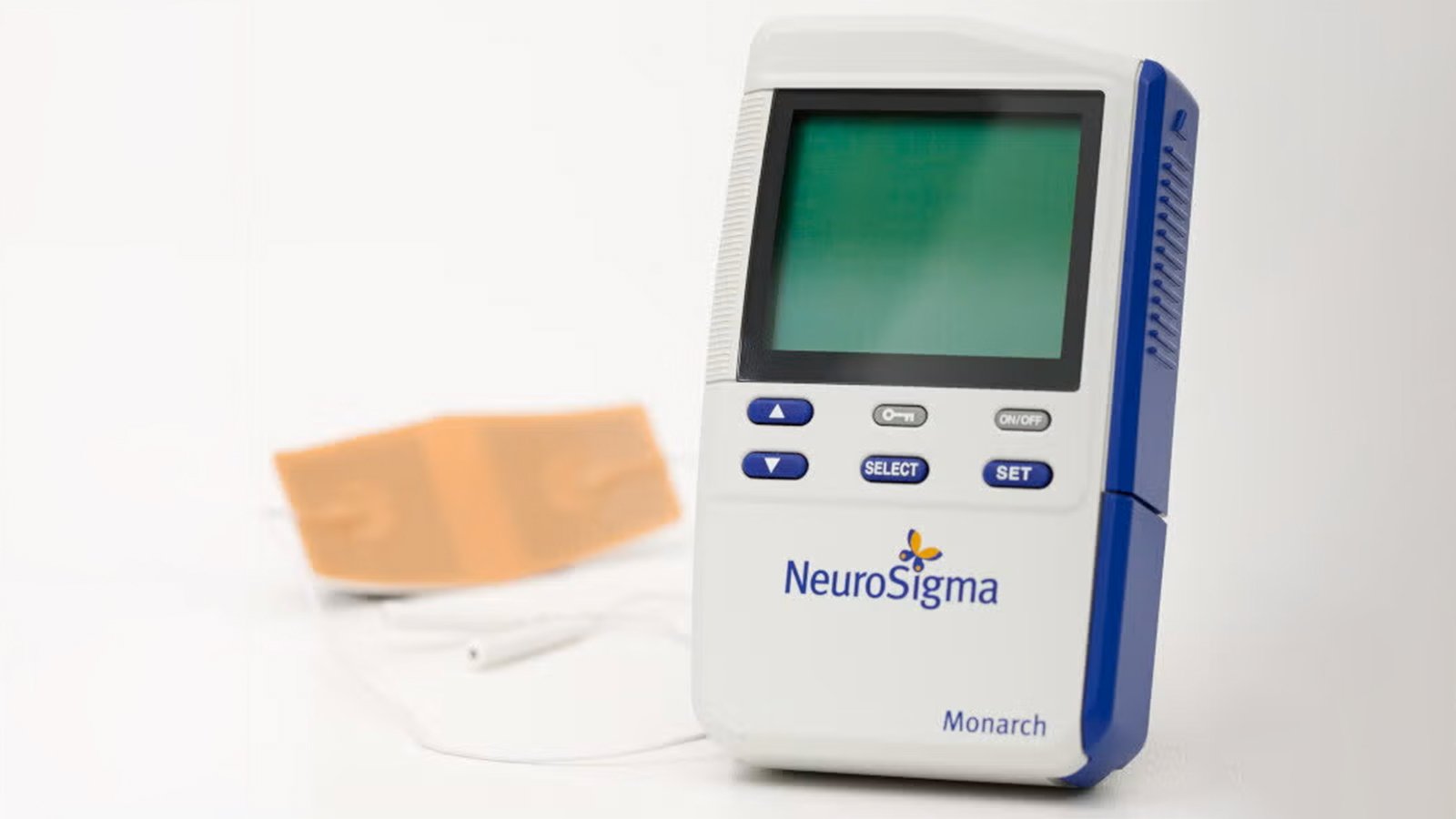
 (MedPage Today) — A phase IIb trial in England suggested that a stimulation device cleared by the FDA in 2019 for children with attention-deficit/hyperactivity disorder (ADHD) may not be effective.
(MedPage Today) — A phase IIb trial in England suggested that a stimulation device cleared by the FDA in 2019 for children with attention-deficit/hyperactivity disorder (ADHD) may not be effective.
In 150 children and adolescents, those treated…
Source link : https://www.medpagetoday.com/pediatrics/adhd-add/119545
Author :
Publish date : 2026-01-22 22:20:00
Copyright for syndicated content belongs to the linked Source.