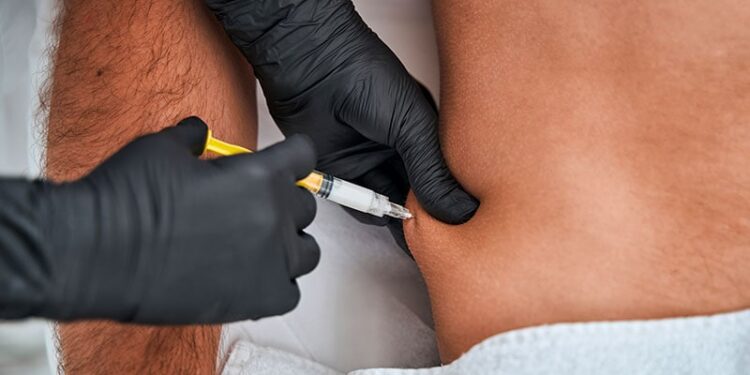
For patients with metastatic non–small cell lung cancer (NSCLC), subcutaneous (SC) administration of pembrolizumab works just as well as standard intravenous (IV) administration, phase 3 data indicated.
Importantly, SC pembrolizumab saves time “potentially streamlining treatment center workflows and lowering healthcare resource utilization,” said Enriqueta Felip, MD, PhD, in a presentation at the European Lung Cancer Congress 2025. The results were simultaneously published in Annals of Oncology.
“This is a novel way of administering pembrolizumab to our patients and the reduced administration time is incredibly impactful,” invited discussant Rosalyn Juergens, MD, PhD, medical oncologist and professor at McMaster University, Hamilton, Ontario, Canada, told attendees.
The open-label, phase 3 MK-3475A-D77 trial enrolled 377 patients with newly diagnosed stage IV NSCLC without sensitizing genetic mutations. They were randomly allocated (2:1) to first-line treatment with pembrolizumab SC (790 mg) or pembrolizumab IV (400 mg) every 6 weeks in addition to platinum doublet chemotherapy.
The study met its dual primary pharmacokinetic endpoints of area under the curve (AUC) of pembrolizumab exposure during the first dosing cycle (0-6 weeks) and steady-state (cycle 3) trough concentration.
Overall exposure and trough concentrations of SC pembrolizumab were on par with IV administration; geometric mean ratios were AUCs of 1.14 for cycle 1 (0-6 weeks) and 1.67 for steady-state trough concentration — above the noninferiority margin of 0.8 (both P < .0001), Felip reported.
The immunogenicity of SC pembrolizumab was low, with no clinically meaningful effect of antidrug antibodies on pembrolizumab exposure. The observed incidence of treatment-emergent antidrug antibodies were 1.4% with SC and 0.9% with IV pembrolizumab.
Efficacy outcomes were “very similar” between the treatment groups, noted Felip, head of the Vall d’Hebron Institute of Oncology, in Barcelona, Spain.
During a median 9.6 months follow-up, objective response rates were 45.4% with SC and 42.1% with IV administration; progression-free survival was 8.1 months and 7.8 months, respectively; and median overall survival was not reached in either group.
There were no major differences in adverse events (AEs) between the two treatment groups. SC pembrolizumab plus chemotherapy had a manageable safety profile that was “generally consistent” with the known adverse effects of pembrolizumab and chemotherapy, Felip noted.
Grade ≥ 3 drug-related adverse events (AEs) were reported in 47.0% of patients in the SC group and 47.6% in the IV group, with similar rates of discontinuations due to drug-related AEs (8.4% vs 8.7%, respectively). Injection site reactions were infrequent (2.4%) and mild in severity.
A Time Saver and Patient’s Prefer It
Of note, said Felip, median administration time for pembrolizumab SC injection was 2 minutes, which is shorter than the typical infusion time of about 30 minutes for IV pembrolizumab.
Other results from the trial presented as a separate poster at the conference showed that SC pembrolizumab (vs IV) reduced time for patients spent in-chair and in the chemotherapy treatment suite by 49.7% and 47.4%, respectively, and reduced the total active time spent by healthcare professionals on treatment preparation, administration process, and patient monitoring by 45.7%.
“As a physician, I am thrilled to see these data for subcutaneous pembrolizumab, which, if approved, has the potential to give patients valuable time back in their treatment day with results that are consistent with IV pembrolizumab,” Felip said in a news release from Merck.
Juergens agreed. “Our chemo day suites are absolutely packed. During weeks that we have holidays, I am having to skip doses of treatment or delay start of treatment. Being able to have more time in those chairs is incredibly important for patients. It not only improves the flow-through of our chemotherapy suites but it’s actually impactful to the pharmacists and the pharmacy technicians,” she told conference attendees.
Based on the data, the US Food and Drug Administration has accepted the company’s Biologics License Application seeking approval of SC pembrolizumab across all previously approved solid tumor indications.
The application has a target action date of September 23, 2025.
This study was supported by Merck Sharp & Dohme LLC. Felip and Juergens have disclosed various relationships with Merck Sharp & Dohme and other pharmaceutical companies.
Source link : https://www.medscape.com/viewarticle/subcutaneous-pembro-par-iv-administration-lung-cancer-2025a10007lc?src=rss
Author :
Publish date : 2025-03-31 07:21:00
Copyright for syndicated content belongs to the linked Source.