TOPLINE:
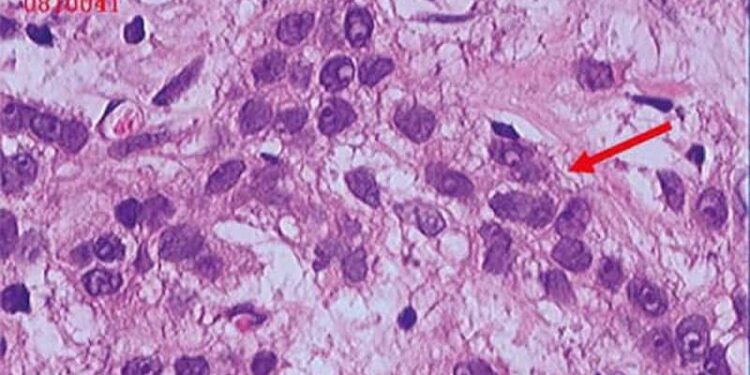
Neoadjuvant chemotherapy (NACT) use in triple-negative breast cancer (TNBC) increased from 19.1% to 56.4% between 2010 and 2021, with pathologic complete response (pCR) rates rising from 19.6% to 40.3%. Black patients showed lower likelihood of receiving NACT and achieving pCR than non-Hispanic White patients.
METHODOLOGY:
- NACT has evolved as the standard treatment for high-risk early invasive TNBC, offering benefits like increased breast conservation rates and prognostic information. Recent developments in adjuvant therapy options, including capecitabine for residual disease and olaparib for BRCA mutations, have provided additional rationale for NACT use.
- Researchers identified patients aged ≥ 18 years with stage I-III TNBC diagnosed between 2010 and 2021 in the National Cancer Database.
- Analysis included assessment of trends in NACT use, pCR, and overall survival using the Cochran-Armitage test.
- Multivariable logistic regression evaluated factors associated with NACT use and pCR achievement.
- The impact of pCR on overall survival was examined using multivariable Cox proportional hazards model with propensity score adjustment and matching.
TAKEAWAY:
- NACT receipt increased significantly from 19.1% to 56.4% between 2010 and 2021, with pCR rates rising from 19.6% to 40.3% (P < .001).
- Three and 5-year overall survival rates improved among patients with residual disease, while remaining stable for those achieving pCR.
- Black patients showed lower likelihood of receiving NACT (adjusted odds ratio [aOR], 0.88; 95% CI, 0.85-0.91) and achieving pCR (aOR, 0.90; 95% CI, 0.85-0.95) compared with non-Hispanic White patients.
- The achievement of pCR was associated with significantly lower risk for death (adjusted hazard ratio, 0.26; 95% CI, 0.24-0.29).
IN PRACTICE:
“The use of NACT among patients with TNBC has dramatically increased in the past decade. Although TNBC is more prevalent in Black patients, they were less likely to be treated with NACT and less likely to achieve a pCR. Further research is needed to elucidate the underlying disparities and advance drug development to enhance outcomes,” the authors of the study wrote.
SOURCE:
This study was led by Mariana Chavez-MacGregor, MD, MSc, FASCO, The University of Texas MD Anderson Cancer Center in Houston. It was published online in JCO Oncology Practice.
LIMITATIONS:
The researchers acknowledged that while the study included a large cohort of TNBC patients, the hospital-based nature of data from the National Cancer Database may limit generalizability. Additionally, as the most recent data available was from 2021, the full impact of the KEYNOTE-522 regimen approval on treatment patterns and outcomes could not be fully captured. The study was also limited in its ability to analyze the early incorporation of the KEYNOTE-522 regimen after its initial presentation in 2019.
DISCLOSURES:
This study received support from the Susan G. Komen Foundation, The Breast Cancer Research Foundation, and the National Cancer Institute at the National Institutes of Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Source link : https://www.medscape.com/viewarticle/outcomes-improve-triple-negative-bc-2025a10007hh?src=rss
Author :
Publish date : 2025-03-28 12:43:00
Copyright for syndicated content belongs to the linked Source.