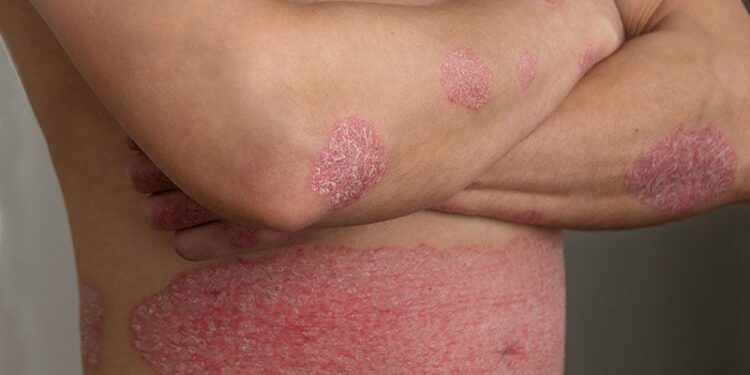
Dermatologists are practicing in an “era of good efficacy” of nonsteroidal topical therapies for psoriasis, and they can help patients access newer topical medications by working with pharmacies that are most familiar with them, advised April W. Armstrong, MD, MPH, during a Grand Rounds lecture held by the Department of Dermatology, The George Washington University, Washington, DC.
While the armamentarium of biologics for treating psoriasis has exploded in recent years, boosting treatment success, and making biologic selection for patients with moderate to severe disease a key issue, recent advances in topical formulations and vehicles have been clinically significant as well, with nonsteroidal topical therapies now an increasingly effective, well tolerated option for nonsevere plaque psoriasis, she said.
Currently available fixed combination topicals have robust efficacy, and the recently approved cream formulations of tapinarof and roflumilast appear to have efficacy that may be higher than previously available nonsteroidal topical calcineurin (TC) inhibitors and similar to mid-to-high potency topical corticosteroids, said Armstrong, professor and chief of dermatology at the University of California, Los Angeles.
During a lecture that addressed oral therapies as well as biologics, Armstrong also noted the potential usefulness of small oral molecules like deucravacitinib either as the primary systemic agent for those with more moderate plaque psoriasis or, in some cases, as an adjunct to biologics for recalcitrant moderate to severe psoriasis.
Fixed Combination Topical Treatments
Topical treatment is increasingly assuming a “proactive maintenance” role—rather than solely reactive — in which areas that frequently flare but are now clinically quiescent are treated once or twice a week to reduce the risk for recurrence, often with a “mix and match” combination of topicals. “Once lesions are clear or almost clear…typically, I would consider nonsteroidal agents in these areas for long-term maintenance therapy,” Armstrong said.
Additionally, today’s menu of fixed combination topical therapies includes agents such as calcipotriene (CAL) and betamethasone dipropionate (BDP) (0.005%/0.064%) foam and cream and halobetasol propionate (HP) and tazarotene (TAZ) lotion (0.01%/0.045%).
The development of the aqueous cream formulation of CAL/BDP, approved for plaque psoriasis in 2020, was a “big deal” because until then, the pH incompatibility of the two active ingredients had meant that formulations needed to be anhydrous or oil-based (a foam, ointment, or suspension). The cream, which is approved for once-daily use, is formulated using polyaphron dispersion (PAD) technology, which creates tiny spheres, or droplets, that “can be packed with different molecules of interest and then combined together,” she said. The droplet-housed active ingredients are both chemically and physically stable, and the droplets require low levels of surfactant, with potential for less irritation.
The CAL/BDP cream formulation has “been very well tolerated and actually has been shown in a head-to-head fashion to have higher efficacy [than a marketed topical suspension as an active control]. The only difference is the vehicle,” Armstrong said, referring to a 2022 pooled analysis of phase 3 trial data.
Treatment success as per the Physician Global Assessment was 43% for the CAL/BDP cream compared with 32% for the suspension/gel (P
“We typically don’t think about putting cream on the scalp,” she added, but additional research looking at this PAD-formulated cream on the scalp has shown good tolerability and feasibility. “I’d thought the foam would be a slam dunk, but I’ve had patients who don’t love certain types of foam formulations in their scalp,” she said. “Patients will tell you what they prefer.”
CAL/BDP foam, which is approved for once-daily use in patients 12 years or older (avoiding the face, groin, and axillae), has rare potential side effects of hypercalcemia and hypercalciuria, but “you’d have to put on a lot to run into this issue in an otherwise healthy person,” Armstrong noted. “Overall, it’s well tolerated.”
The fixed-combination HP/TAZ lotion also has robust efficacy, she continued. In phase 3 randomized controlled trials, she noted, HP/TAZ lotion achieved up to 36%-45% treatment success over 8 weeks (at least a 2-grade improvement from baseline in Investigator’s Global Assessment score and a score of clear or almost clear compared with 7%-12.5% success with placebo/vehicle in patients with moderate to severe psoriasis.
HP/TAZ lotion must be applied in a thin layer, she cautioned. “This is the key. Applying it correctly with a thin, not a thick, layer can minimize the potential risk of contact dermatitis. A little goes a long way.”
The active ingredients in the lotion are believed to have a synergistic effect, with the topical corticosteroid reducing tazarotene-induced irritation, and tazarotene mitigating corticosteroid safety concerns and increasing the duration of treatment effects.
Tapinarof, Roflumilast
A naturally occurring, small molecule aryl hydrocarbon receptor (AhR) agonist, tapinarof has an intriguing backstory: It’s a metabolite of a bacterium, Photorhabdus luminescens, which lives symbiotically in the intestines of nematodes (roundworms). By modulating the AhR, tapinarof 1% cream has a unique mechanism of action that leads to increased antioxidant activity via the nuclear factor erythroid 2–related factor 2 pathway and as a direct free radical scavenger; improved skin barrier function through increased filaggrin, loricrin, and involucrin; and decreased inflammation.
“Two of the important downstream effects [of tapinarof] are its effects on the Th17 pathway, which is why we have approval in psoriasis, and on the Th2 pathway, which is why it’s being studied in AD [atopic dermatitis] and will likely be approved for that as well,” Armstrong said. Tapinarof cream was approved in May 2022 for treatment of mild, moderate, or severe plaque psoriasis in adults.
As per the pivotal PSOARING trials, “about 40% of patients on tapinarof will achieve PASI 75 by week 12, so this is very good for a topical nonsteroidal,” Armstrong said.
And in the longer term, as per the PSOARING open-label extension study, “for patients who clear, it takes about 4 months [off treatment] before they experience mild severity,” she said. “So there is potentially a long-term remittive effect, which is helpful in the real world.”
In a small proportion of patients, the use of tapinarof cream may be associated with folliculitis, which is “more of a keratosis pilaris–like reaction on the skin,” Armstrong said. While few patients may experience contact dermatitis, most tolerate the cream very well.
Roflumilast, a topical phosphodiesterase 4 (PDE4) inhibitor, was approved for the treatment of plaque psoriasis in adults and adolescents in 2022, and in children aged 6 years or up in 2023. While there are no head-to-head comparisons with tapinarof, the pivotal phase 3 DERMIS clinical trial data for roflumilast appeared to be similar, with about 40% achieving investigator global assessment treatment success by week 8.
“One of its key advantages is its good tolerability and language on the label about using it in intertriginous areas,” she said. “Especially for sensitive areas, topical roflumilast is a good choice.”
Nonsteroidal topicals are advantageous in that they can be used long-term. “The key, however, is access,” Armstrong said. The newer topicals require preapproval that is contingent on documented failure of topical steroids and TC inhibitors, and “knowing where to send the prescription is really important,” she said. “Oftentimes, smaller local pharmacies are more familiar with newer topical medications.”
Oral Small-Molecule Drugs: Possible Add-On to Biologics for Recalcitrant Disease
The first-in-class tyrosine kinase 2 (TYK2) inhibitor deucravacitinib, approved in 2022 for adults with moderate to severe psoriasis, was shown to be superior to the oral PDE4 inhibitor apremilast in the POETYK PSO pivotal trials, with nearly 54% achieving PGA 0/1 (clear or almost clear) at 16 weeks and 60% of patients at 24 weeks compared with 32% and 31%, respectively, on apremilast, Armstrong said. (The trials also measured superior PASI-75 and PASI-90 responses with deucravacitinib.)
Moreover, long-term maintenance data show that “about 75% of patients maintained a PASI-75 response at 4 years,” she said. “If you start a patient on deucravacitinib, the likelihood of maintaining that response is pretty good.”
The selective TYK2 inhibitor interferes with signaling of interleukin (IL)-23, IL-12, and type 1 interferons. It was approved for treatment of moderate to severe plaque psoriasis, but the once-daily 6 mg tablet “shines the most” for moderate (not extensive) disease “and is well tolerated by patients,” Armstrong said.
Additionally, she said, consideration can be given to adding deucravacitinib to a biologic for recalcitrant disease. “In those rare patients who still have moderate disease after you’ve maximized their biologic, you may want to think about a biologic and deucravacitinib,” Armstrong said.
She shared the case of a 60-year-old man who presented to her clinic with his “skin on fire,” with severe pruritus and burning; erythema covering 90% of his body; significant desquamation on the upper body, back, and bilateral upper extremities; significant nail dystrophy in the fingernails; and hair loss. The patient’s history included evaluation for eczema and unsuccessful treatment with dupilumab, and a trial of cyclosporin (when the patient was previously thought to have eczema). When presenting to Armstrong’s clinic, the patient was on mycophenolate mofetil and prednisone.
A biopsy at Armstrong’s clinic showed erythrodermic psoriasis with diffuse arthralgia. She started him on an IL-17 inhibitor and weaned him off the prednisone and mycophenolate mofetil. At the 3-month follow-up, he had significant improvement but was “still not completely better, so I decided to add deucravacitinib,” she said. “Three months later, the erythema was pretty much gone, the scales had also disappeared, and his nails were improved.”
During a discussion period, Adam Friedman, MD, professor and chair of dermatology at The George Washington University, said the oral small molecule inhibitors can indeed be helpful add-ons to biologics, including in cases appearing to involve some immune drift toward a more eczematous picture.
Such combination treatment can be “a struggle with payors,” Armstrong said. “We need to spend more time in working with insurance companies closely, documenting the difficult journey these patients have had, and why the patient may benefit from certain regimens.”
Armstrong disclosed that she is a research investigator and/or scientific advisor to AbbVie, BMS, Dermavant, Galderma, Incyte, Janssen, Leo, Lilly, Novartis, OrthoDerm, Pfizer, Regeneron, Sanofi, Sun, and UCB. She had served as a principal investigator in clinical trials at her academic institution for the development of most topicals and systemic medications discussed in the story. All clinical trial funding goes to the academic institution.
Source link : https://www.medscape.com/viewarticle/topical-psoriasis-therapies-tapinarof-roflumilast-and-newer-2025a10004sz?src=rss
Author :
Publish date : 2025-02-25 11:08:26
Copyright for syndicated content belongs to the linked Source.