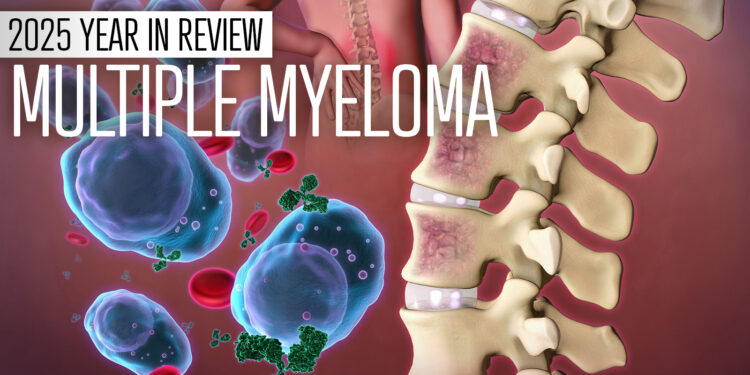
 (MedPage Today) — News involving the treatment of multiple myeloma this year was highlighted by talk of a potential cure with a single infusion of a chimeric antigen receptor (CAR) T-cell therapy, new FDA approvals (including a drug once pulled…
(MedPage Today) — News involving the treatment of multiple myeloma this year was highlighted by talk of a potential cure with a single infusion of a chimeric antigen receptor (CAR) T-cell therapy, new FDA approvals (including a drug once pulled…
Source link : https://www.medpagetoday.com/hematologyoncology/myeloma/118278
Author :
Publish date : 2025-11-03 17:52:00
Copyright for syndicated content belongs to the linked Source.